




Search websites, locations, and people

New Catalyst Delivers Industry-Grade Formic Acid from CO2
27, 2023
Email: sangeet@westlake.edu.cn
Phone: +86-(0)571-88112035
Office of the Dean, School of Science
A team led by Prof. Zhang Biaobiao at the Center of Artificial Photosynthesis for Solar Fuels of Westlake University has developed a new catalyst that can help produce industrial-grade formic acid from carbon dioxide, offering an innovative solution to convert the greenhouse gas into fuel and mitigate global warming.
Formic acid is a promising chemical that can be used for liquid hydrogen storage and formic acid fuel cells in moving toward a carbon-free future. However, the conversion of carbon dioxide into formic acid at an industry-grade level is not without its challenges.
To address these obstacles, in a recent study, Prof. Zhang’s team developed a new class of catalyst consisting of indium cyanamide nanoparticles and employed it for the electroreduction of carbon dioxide. The catalyst prevented self-reduction under carbon dioxide reduction conditions, enabling long-term electrocatalysis based on the indium oxidation state, and demonstrated outstanding consistency in pure formic acid production from carbon dioxide at industrial-grade current density and stability.
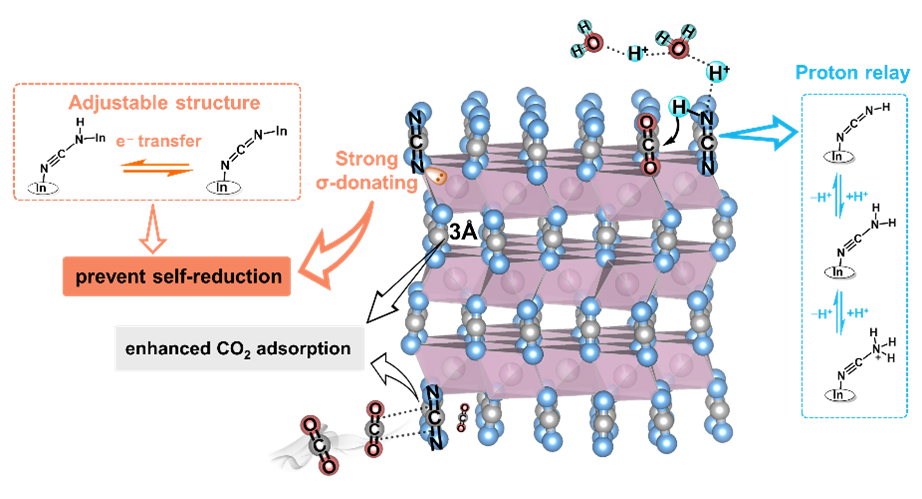
Figure 1. Schematic illustrations of InNCN structural advantages for CO2 reduction.
The study has affirmed the great potential of metal cyanamides in carbon dioxide reduction, broadening the understanding of the structure-activity relationships of the carbon dioxide reduction catalysts, and expanding the design strategies of effective catalysts.
The study was published in the prestigious Journal of the American Chemical Society, under the title “Indium Cyanamide for Industrial-Grade CO2 Electroreduction to Formic Acid.” Jia Bingquan, a postdoctoral fellow of Westlake University, is the first author while the doctoral candidate Chen Zhe is the joint first author. Dr. Zhang Biaobiao, PI of Westlake University, is the independent corresponding author.
Electrochemical CO2 reduction to chemicals, such as CO, HCOOH, CH3OH, ethylene, and ethanol, is a promising one-stone-two-birds strategy for carbon neutrality, resulting in both "carbon negative" and green fuels. A number of different electrocatalysts have been reported to achieve high single-product selectivity and Faradaic efficiency. However, it remains a significant challenge to achieve industrial-grade current density and stability, requiring the development of new materials for more efficient and durable electrochemical CO2 reduction. Metal cyanamides with unique structural features, including strongly σ-donating [NCN]2− ligands, the potential structural transformation of [N=C=N]2− and [N≡C−N]2−, and the open framework structure, are promising novel materials for electrocatalytic CO2 reduction, broadening the variety of CO2 reduction catalysts and bringing new chances for approaching industrial-grade CO2 reduction techniques.
RELATED
NEWS
New Catalyst Delivers Industry-Grade Formic Acid from CO2




















