




Search websites, locations, and people

Zhaobin Wang's Group Develops Promising Method to Facilitate Non-Standardized Carbene-based Asymmetric Synthesis
05, 2023
Email: sangeet@westlake.edu.cn
Phone: +86-(0)571-88112035
Office of the Dean, School of Science
Professor Zhaobin Wang and his team from the School of Science at Westlake University have made significant progress in the asymmetric cyclopropanation of alkenes using gem-dihaloalkanes as carbene precursors catalyzed by chromium.
Their findings have been published in the esteemed journal Applied Chemistry Germany in a paper titled “Catalytic Diastereo- and Enantioselective Cyclopropanation of gem-Dihaloalkanes and Terminal Olefins.”
By employing gem-dihaloalkanes, the authors successfully overcame the limitations posed by non-resonant stabilized diazo alkanes in asymmetric carbene transfer reactions. This groundbreaking achievement establishes a highly efficient synthetic methodology for the production of multiply substituted chiral cyclopropane compounds. Additionally, through an extensive array of mechanistic studies encompassing radical capture experiments, nonlinear effects experiments, and UV-visible spectroscopy experiments, the authors conducted preliminary investigations into the potential reaction intermediates, providing valuable supportive insights into the catalytic process.
Cyclopropane, characterized by its high strain and structural rigidity, exhibits distinctive properties. The cyclopropane motif finds widespread presence in natural products, pharmaceuticals, and biologically active compounds (Figure 1a). Furthermore, this structural motif serves as a pivotal building block for crucial synthetic transformations. Consequently, chemists have devised a range of efficient synthetic methodologies for the synthesis of chiral cyclopropanes, including metal- or enzyme-catalyzed cyclopropanation of diazo compounds, Simmons-Smith reaction, Kulinkovich reaction, and desymmetrization of cyclopropanes or cyclopropenes. Among these methodologies, asymmetric carbene transfer reactions between transition metal-catalyzed diazo compounds and alkenes have emerged as one of the most efficient and valuable approaches (Figure 1b). However, the high energy and limited stability of diazo compounds necessitate the reliance on resonance groups (such as ester and cyano) to stabilize diazo compounds in the reported methodologies. Consequently, the achievement of asymmetric cyclopropanation using non-stabilized diazo compounds as carbene precursors remain scarce and poses a significant challenge (Figure 1b).
Gem-dihaloalkanes, renowned for their stability and ready availability, facilitate the generation of zinc carbenoid intermediates through their reaction with zinc and diiodomethane in the classic Simmons-Smith reaction. Subsequently, these intermediates undergo cyclopropanation with alkenes. Building upon this foundation, chemists have developed a variety of gem-dihaloalkanes mediated cyclopropanation reactions involving alkenes, further expanding the realm of application for metal-catalyzed carbene transfer cyclopropanation reactions. Nonetheless, when compared to the asymmetric cyclopropanation of diazo compounds and alkenes catalyzed by transition metals, the current gem-dihaloalkanes mediated cyclopropanation reactions predominantly yield non-chiral cyclopropanes, thereby rendering the attainment of catalytic asymmetric cyclopropanation a challenging endeavor. To address this limitation, the authors introduced a radical-polar crossover strategy, enabling the achievement of asymmetric cyclopropanation of alkenes using gem-dihaloalkanes as carbene precursors, with chromium acting as the catalyst under ligand control (Figure 1c).
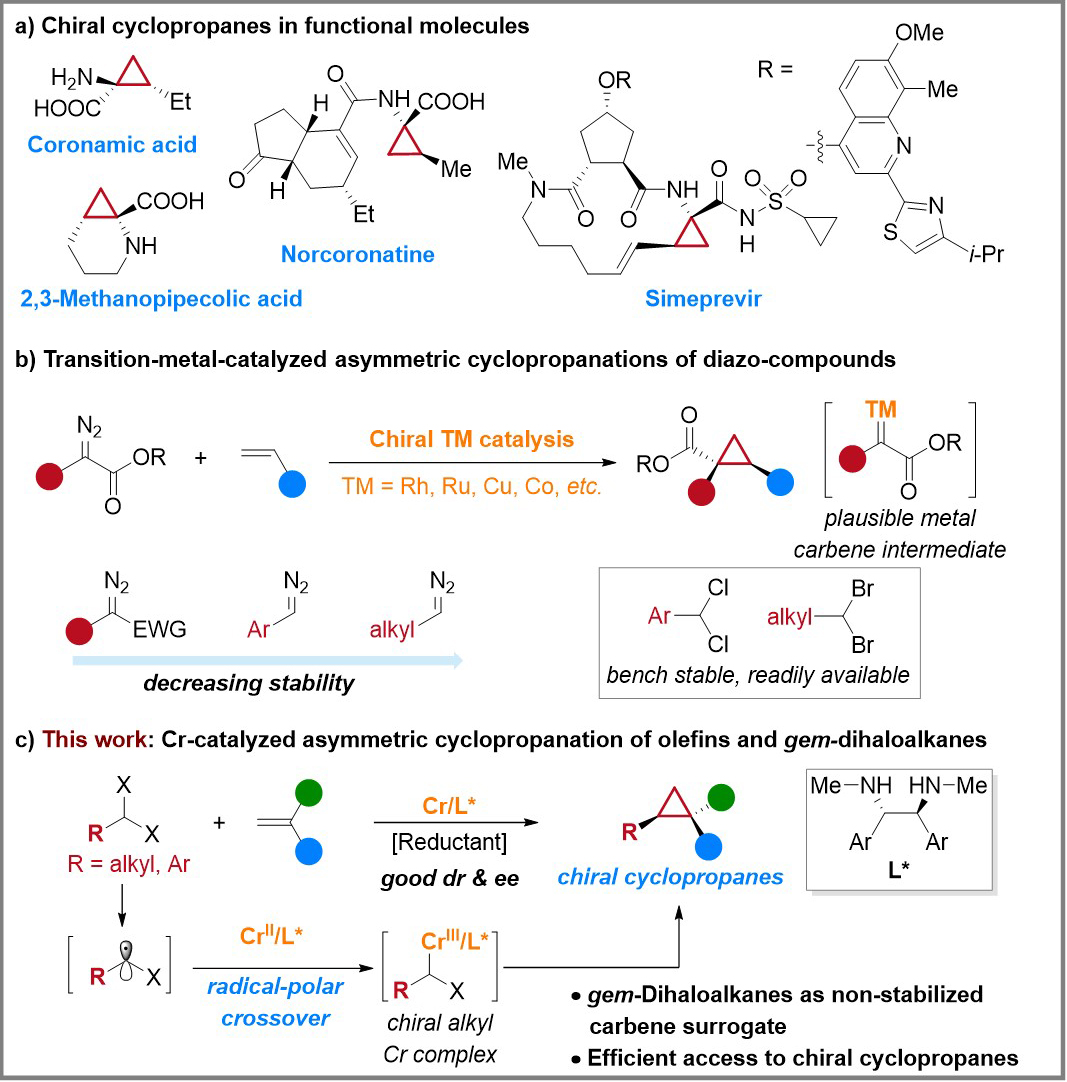
Figure 1. Research background
The authors conducted extensive investigations and established optimized conditions utilizing chiral chromium/diamine catalysts as crucial reaction parameters. Their research primarily focused on investigating the substrate generality of gem-dihaloalkanes and alkenes. The results, depicted in Figure 2, demonstrate the compatibility of gem-dihaloalkanes and 2-substituted acrylamides as substrates in the developed cyclopropanation method. This compatibility extends to a broad range of functional groups, resulting in favorable reaction outcomes. Notably, this methodology was successfully applied in gram-scale experiments, where the resulting products can be converted into chiral cyclopropyl-α-amino acid derivatives through hydrolysis-based deprotection, underscoring the practicality of this approach.
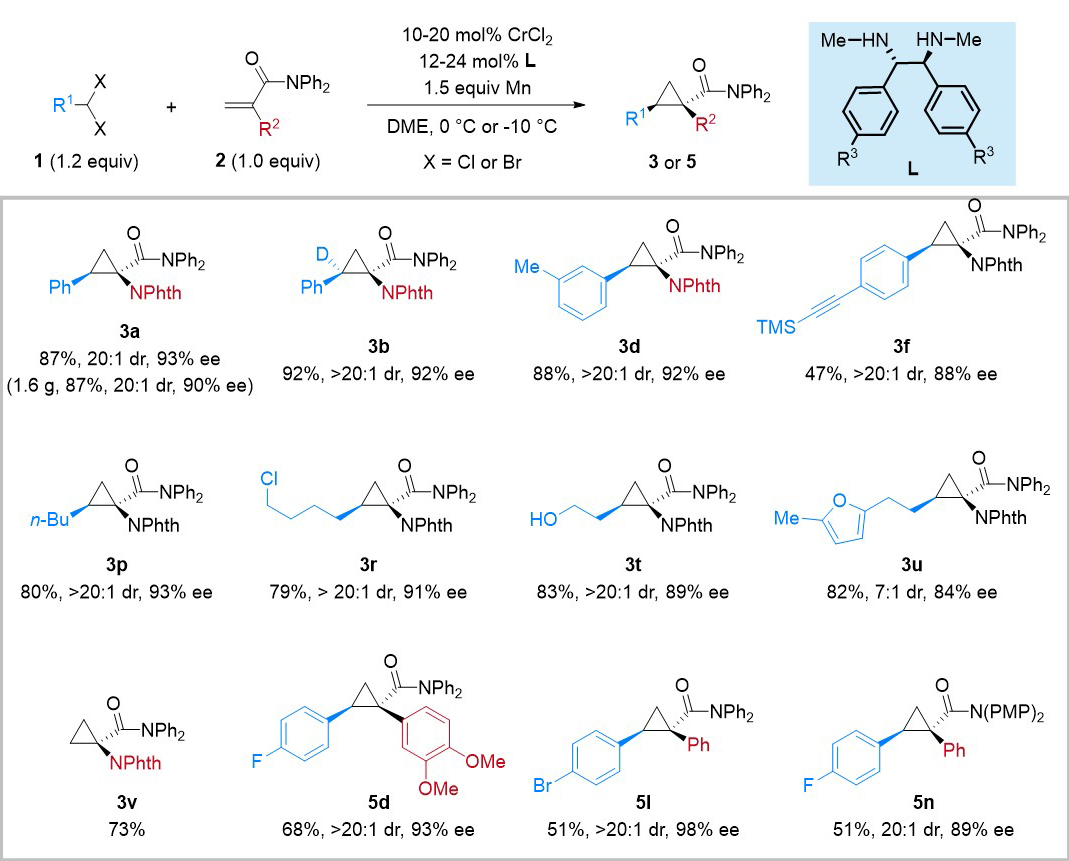
Figure 2. Representative reaction scope
Based on radical capture experiments, nonlinear effects experiments, UV-visible spectroscopy experiments, and relevant literature reports, the authors propose a plausible reaction mechanism (Figure 3). Initially, substrate 1a undergoes reduction by CrII, leading to the formation of the corresponding radical species. This radical species then undergoes a radical-polar crossover with CrII in the system, generating alkyl chromium intermediate B. Regarding the formation of the desired products, the authors propose two potential pathways. In pathway a, alkyl chromium intermediate B undergoes reduction by CrII, resulting in the formation of a dinuclear chromium complex C. This complex subsequently reacts with substrate 2a, leading to the generation of a possible chromium carbene intermediate D. Subsequently, a formal [2+2] cycloaddition reaction takes place, yielding chromium-containing tetrahydrofuran intermediate E. Finally, through a reductive elimination process, intermediate E produces cyclopropane product 3a while simultaneously regenerating the CrII catalyst. In pathway b, alkyl chromium intermediate B interacts with substrate 2a via a concerted transition state F, directly yielding cyclopropane product 3a. The regeneration of the CrII catalyst is facilitated by the reduction with Mn powder. Additionally, α-chloro radicals add directly to 2a, generating a novel radical species. This species subsequently interacts with A and undergoes a concerted cyclization process, ultimately leading to the formation of the product, suggesting a plausible mechanism.
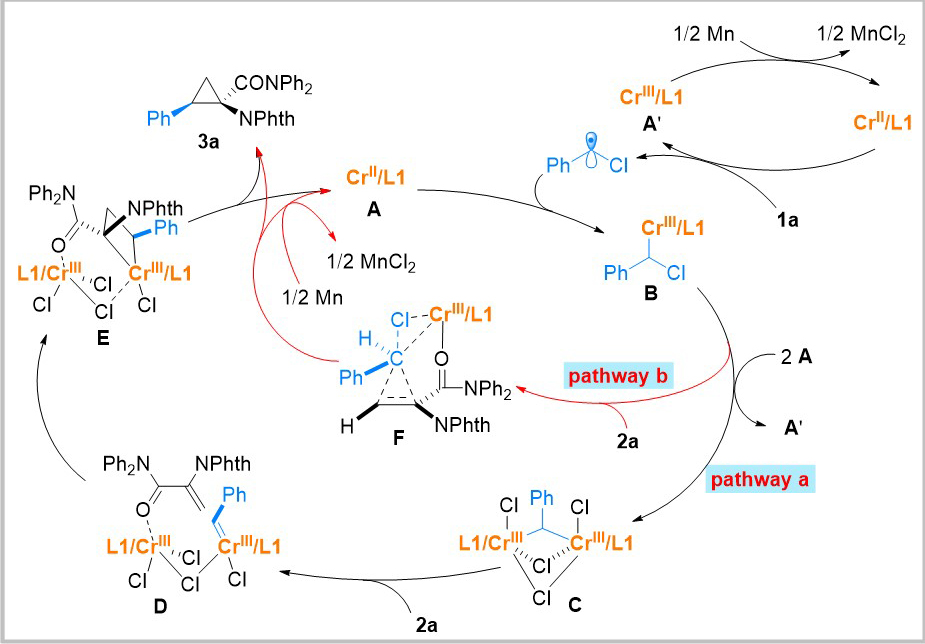
Figure 3. Propose reaction mechanism
In this study, Professor Zhaobin Wang's team achieved a significant milestone by developing the first chromium-catalyzed cyclopropanation reaction between gem-dihaloalkanes and terminal alkenes, demonstrating both non-stereoselective and stereoselective properties. This accomplishment expands the scope of metal-catalyzed carbene transfer cyclopropanation reactions. The success of this method critically relies on the utilization of chiral chromium/diamine catalysts and the presence of 2-substituted acrylamide substrates. Importantly, this approach provides a convenient and efficient means for constructing optically pure cyclopropane compounds, which holds particular relevance in the fields of life sciences and functional molecule synthesis.
To learn more about Prof. Zhaobin Wang's research group and their work, click here.
RELATED
NEWS
New Method Accelerates Peptide Hydrogel Discovery, Opening New Avenues for Medical Applications
NEWS
Zhaobin Wang's Group Develops Promising Method to Facilitate Non-Standardized Carbene-based Asymmetric Synthesis




















