




Search websites, locations, and people

Research opens new possibilities for achieving near-ambient conditions ammonia synthesis from computations
14, 2023
Email: sangeet@westlake.edu.cn
Phone: +86-(0)571-88112035
Recently, Wang Tao’s team from the Center of Artificial Photosynthesis for Solar Fuels and Department of Chemistry at Westlake University published two papers on computational catalyst design for ammonia synthesis. In the JACS Au paper (https://doi.org/10.1021/jacsau.3c00546), titled “Theoretical Approach toward a Mild Condition Haber–Bosch Process on the Zeolite Catalyst with Confined Dual Active Sites”(Chunli Liu, Gaomou Xu, and Tao Wang), they introduced a computational strategy employing dual molybdenum (Mo) active sites within a zeolite framework to effectively activate the inert N2 molecule, paving a solid basis to conduct the ammonia synthesis at near-ambient conditions. Based on his systematic research in ammonia synthesis, Dr. Tao Wang was invited to contribute a comment on this topic to the journal “Nature Computational Science” (https://www.nature.com/articles/s43588-023-00556-6), titled “Fewer False Positives in Electrocatalytic Nitrogen Reduction by Synergizing Theory and Experiment” (Gaomou Xu, and Tao Wang), where theydiscussed the persistence of false positives in nitrogen reduction research and the potential for computational tools to help alleviate this issue.

Scheme 1. This work was selected as the supplementary cover in JACS Au.
As a vital chemical with applications in agriculture, industry, and carbon-free fuels, ammonia (NH3) is predominantly synthesized through the century-old Haber-Bosch (H-B) process. Notably, N2 dissociation was identified as a crucial elementary step on the iron-based catalyst during this process. However, the H-B process faces challenges like high temperature (300~500 ℃) and pressure (150~200 bar), as well as the usage of grey hydrogen, leading to substantial energy consumption and CO2 emissions. Scientists have focused on designing efficient catalysts and exploring innovative reaction mechanisms to develop an ambient conditions H-B process.
Based on Wang Lab’s previous research findings in exploring the mechanism of ammonia synthesis and designing effective catalysts, they chose the ferrierite (FER) zeolite with a suitable pore size as the framework to anchor dual metal active sites, which was capable of addressing the challenge of activating the stable N≡N bond at low temperatures. This innovative catalyst design allows simultaneous bonding of N2 molecules on both sides, activating the stable N≡N bond through enhanced -back-donation (Figure 1). Interestingly, they found that not all the transition metals could achieve this goal, where only the dual Mo active sites worked well in activating N2. In the bridge binding configuration [Mo(II)(NN)Mo(II)], the N≡N bond was elongated from 1.11 Å to 1.21 Å. The calculated energy barrier for direct N≡N bond breaking is only 0.58 eV, demonstrating the catalyst’s high feasibility to conduct ammonia synthesis under mild conditions.

Figure 1. (A) The model structure of designed 2Mo(II)-FER catalyst; (B) Enhanced -back-donation schematic.
For the hydrogenation steps, unlike the traditional metal-based catalysts, the uniquely designed 2Mo(II)-FER catalyst has three different hydrogen sources shown in Figure 2, i.e., the BH within the zeolite, the H near the N on the Mo(II) site (NMH) and the H far from the N on the other Mo(II) site (FMH), which greatly enrich the hydrogenation mechanisms. Specifically, for the NH intermediate, the FMH pathway provides an ideal structure for the overlap of the s orbital of H with the frontier orbital of the N, resulting in the low energy barrier. Finally, the three distinct hydrogen sources showed superior potential with a hydrogenation barrier of less than 0.69 eV. The catalyst demonstrated very high efficiency in ammonia synthesis, showcasing a breakthrough in catalyst design and providing new insights for alternative hydrogenation reaction pathways.
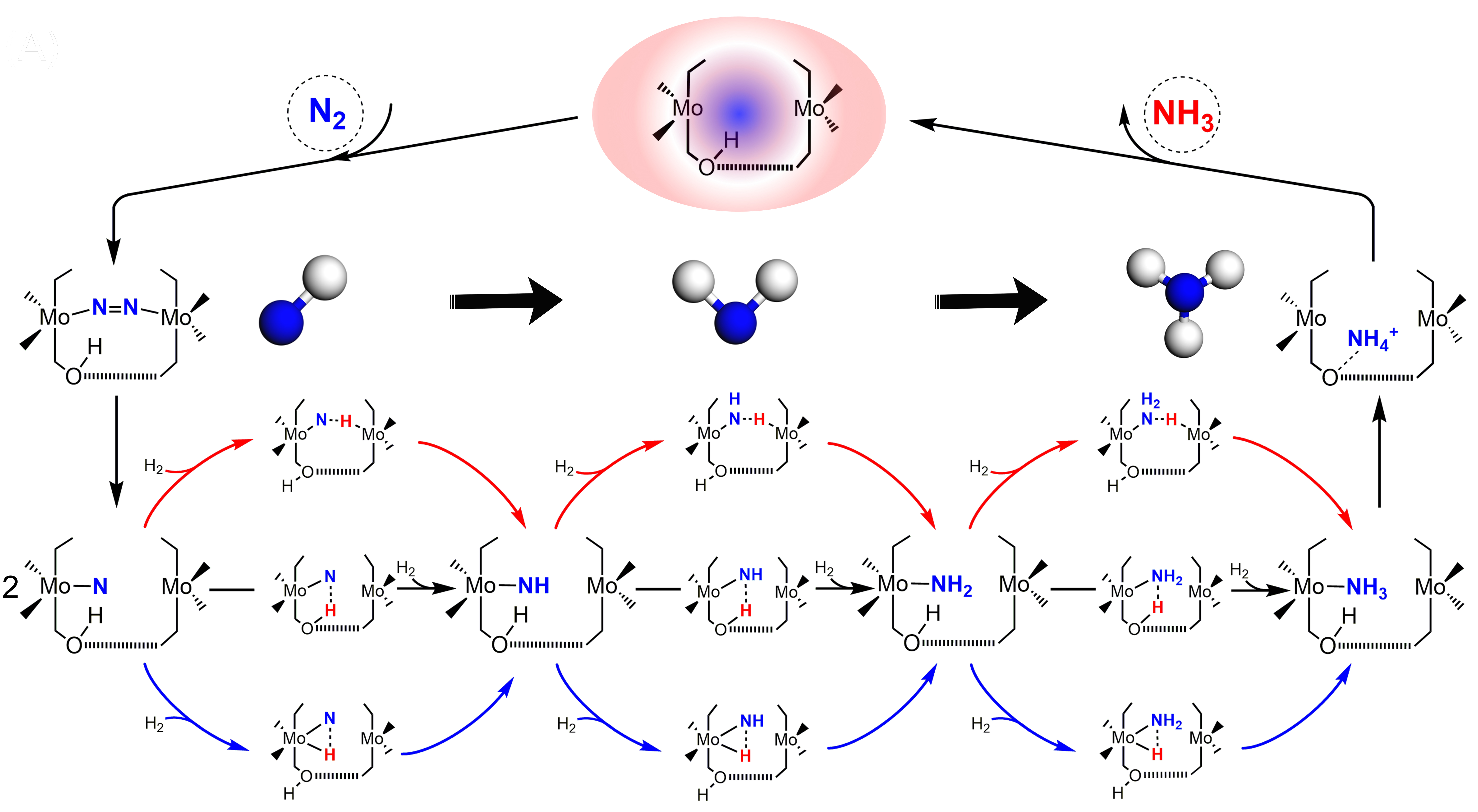
Figure 2. Reaction mechanism of the H-B process with different H sources
To further evaluate the reaction rate of the 2Mo(II)-FER catalyst for ammonia synthesis, they established a mean-field microkinetic model (MKM) to calculate the TOFNH3 under different reaction conditions. For a convenient benchmark, the stepped (211) surface of FCC Ru, recognized as a highly active metal for ammonia synthesis, was chosen. Notably, the newly designed catalyst demonstrated a much higher TOF for ammonia synthesis under the same conditions, with four orders of magnitude higher than the Ru(211) surface at 500 K and 100 bar (Figure 3). These simulation results strongly indicate the potential for achieving a near-ambient conditions H-B process with this innovative 2Mo(II)-FER catalyst. Moreover, the use of zeolite as the carrier enhanced composability in the laboratory and provided a crucial theoretical reference for the realization of the ammonia synthesis process at low temperatures.
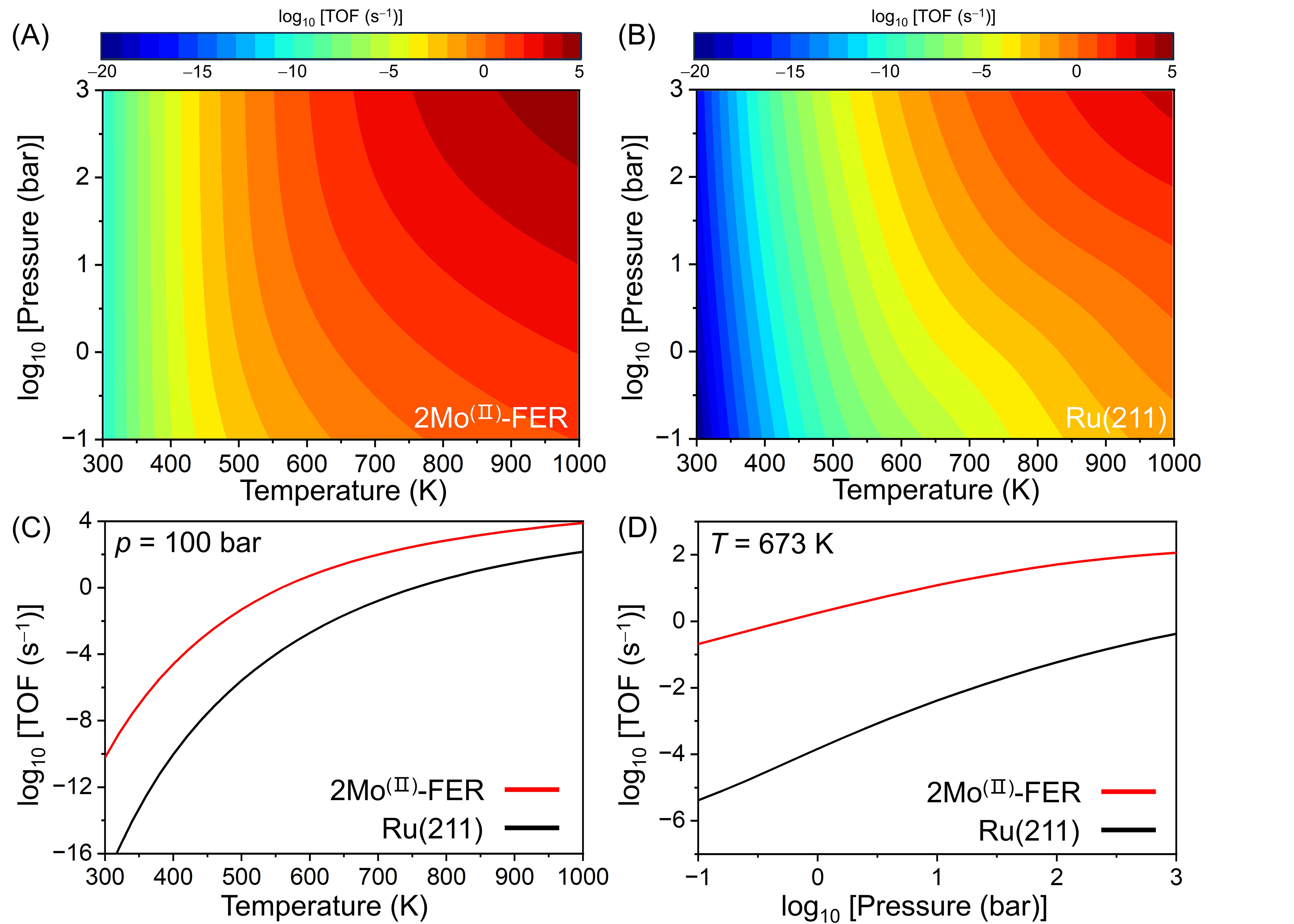
Figure 3. Calculated TOF of NH3 production at different conditions on the 2Mo(II)-FER and stepped Ru(211) surface
Actually, Wang Tao’s team is very interested in computational catalyst design and proposing new understandings of reaction mechanisms for ambient condition ammonia synthesis with both thermal catalysis and electrocatalysis. Based on their recent research works at Westlake University[1-6], Dr. Tao Wang was invited to contribute a comment on this topic. Focusing on the widespread problem of false positives in eNRR, they discussed the limitations of existing experimental and computational methodologies and proposed an integrated theoretical and experimental framework, which introduced multi-system comparisons to identify false positives in research work (Figure 4). The expansion of this framework as well as the development of advanced computational methods could be a promising route to enhance the reliability and repeatability of highly efficient eNRR catalysts design. At last, they emphasized the importance of synergizing theory and experiment in achieving rational catalyst design for effective nitrogen fixation. Their insights were shared in an invited comment in “Nature Computational Science” (Gaomou Xu and Tao Wang), titled “Fewer false positives in electrocatalytic nitrogen reduction by synergizing theory and experiment”.
DOI:https://www.nature.com/articles/s43588-023-00556-6
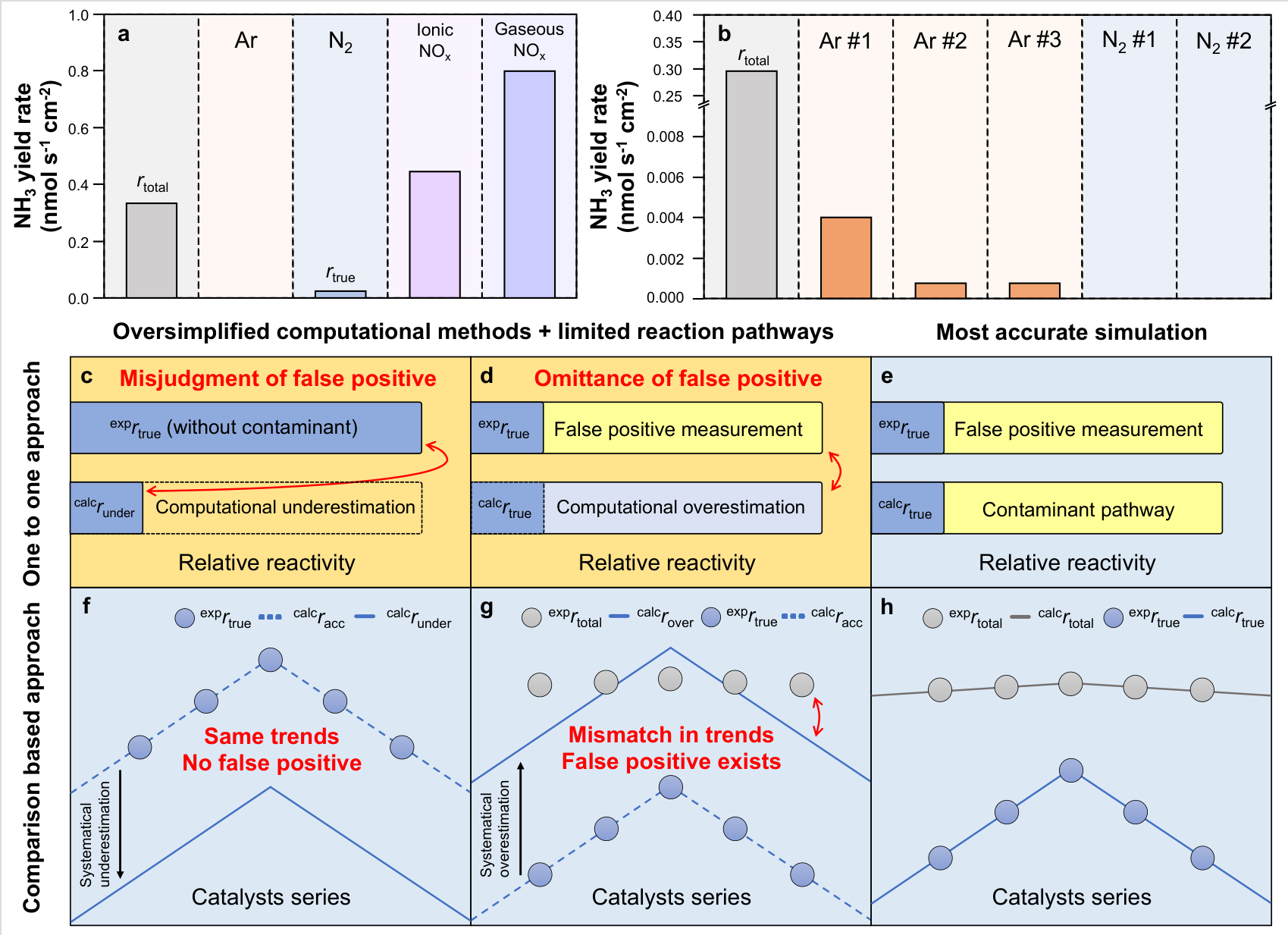
Figure 4. The framework to identify the false positives in research work
Acknowledgment
These works were supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, and the Research Center for Industries of the Future (RCIF) at Westlake University. Westlake University HPC Center for Computation support.
References
(1) Xu, G.; Wang, T.* Nat. Comput. Sci. 2023, https://doi.org/10.1038/s43588-023-00556-6.
(2) Liu, C. L.†; Xu, G.†; Wang, T.* JACS Au, 2023, https://doi.org/10.1021/jacsau.3c00546.
(3) Zhao, W. H.†; Xu, G.†; He, Z. C.; Cai, C.; Abild-Pedersen, F.; Wang, T.* J. Am. Chem. Soc. 2023, 145, 8726.
(4) Xu, G.†;Cai, C.†; Wang, T.* J. Am. Chem. Soc. 2022, 144, 23089.
(5) Chen, Z.†; Liu, C. L.†; Sun, L. C.; Wang, T.* ACS Catal., 2022, 12, 8936.
(6)Wang, T.*; Abild-Pedersen, F.* Proc. Natl. Acad. Sci.2021, 118, e2106527118.
RELATED
NEWS
Research opens new possibilities for achieving near-ambient conditions ammonia synthesis from computations




















