




Search websites, locations, and people

Innovative Phenazine-based Carbon Capture Cell Developed
26, 2023
Email: zhangchi@westlake.edu.cn
Phone: +86-(0)571-86886861
Office of Public Affairs
Prof. Pan Wang’s Lab at Westlake University recently published a paper in the journal Nature Energy, titled “A Phenazine-based High-Capacity and High-Stability Electrochemical CO2 Capture Cell with Coupled Electricity Storage”. The study focused on energy storage in water-based organic batteries that can also be used in CO2 capture systems.
The paper can be read in full at https://www.nature.com/articles/s41560-023-01347-z.
The average global temperature today is more than 1oC warmer than before the industrial revolution. Greenhouse gas emissions are the primary drivers of climate change, and accumulated CO2 from fossil fuel consumption is the major source of global warming, ocean acidification, and other severe environmental problems. In addition to the urgent replacement of fossil energy by clean energy, CO2 capture is also vital because fossil fuel combustion will inevitably remain prevalent for some time to come, and pollutants that are emitted now will still be present in the environment even once the electricity sector is fully decarbonized. To keep the global temperature increase below 1.5oC in this century, significant progress needs to be achieved in efficient, large-scale CO2 capture techniques, whether from point sources such as a fossil fuel combustion, from biomass power plants, or directly from the atmosphere.
Electrochemically mediated CO2 capture may provide a relatively low-cost, environmentally benign, and less energy-intensive approach, as it can be operated at ambient temperature and pressure without relying on external energy sources. CO2 capture based on electrochemically induced pH swing, in which CO2 is absorbed at high pH and released at low pH, is a promising carbon capture method because of its low energy requirements and high current density. The necessary pH swing can be driven electrochemically through proton-coupled electron transfer (PCET) of organic molecules. During electrochemical reduction and oxidation, molecules undergoing PCET reactions capture and release protons, leading to pH decrease and increase, respectively, of the solution. Leveraging the high tunability of redox-active organic molecules, electrolytes can be produced with the desired redox potential, high solubility, long lifetimes, and low cost.
Fig. 1 shows a schematic illustration of an integrated CO2 capture-release and energy storage-delivery system using 1,8-ESP. In the charging process, 1,8-ESP on the negative side, paired with ferrocyanide on the positive side (Fig. 1a), undergoes a two-electron reduction process, with its reduced form – re-1,8-ESP – rapidly consuming two protons from H2O, which increases the pH and total alkalinity (TA) of the solution. This process is called deacidification. Gas containing CO2 reacts with OH‑ to form dissolved inorganic carbon (DIC), completing the CO2 absorption and energy storage half-cycle. During discharging (Fig. 1b), re-1,8-ESP is oxidized to 1,8-ESP, releasing protons into the solution. This process is called acidification. The pH and TA of the electrolyte both decrease, resulting in outgassing of pure CO2. Electrical energy delivery and pure CO2 gas release therefore occur simultaneously during this half-cycle. For commercial operators, this means that capital costs can be shared, while providing storage for the electrical grid at the same time. Electricity price arbitrage in an electricity market is only profitable a small fraction of the time, so dedication of an integrated system to CO2 capture during periods when arbitrage is unprofitable could lead to significantly improved economics over operating either process alone.
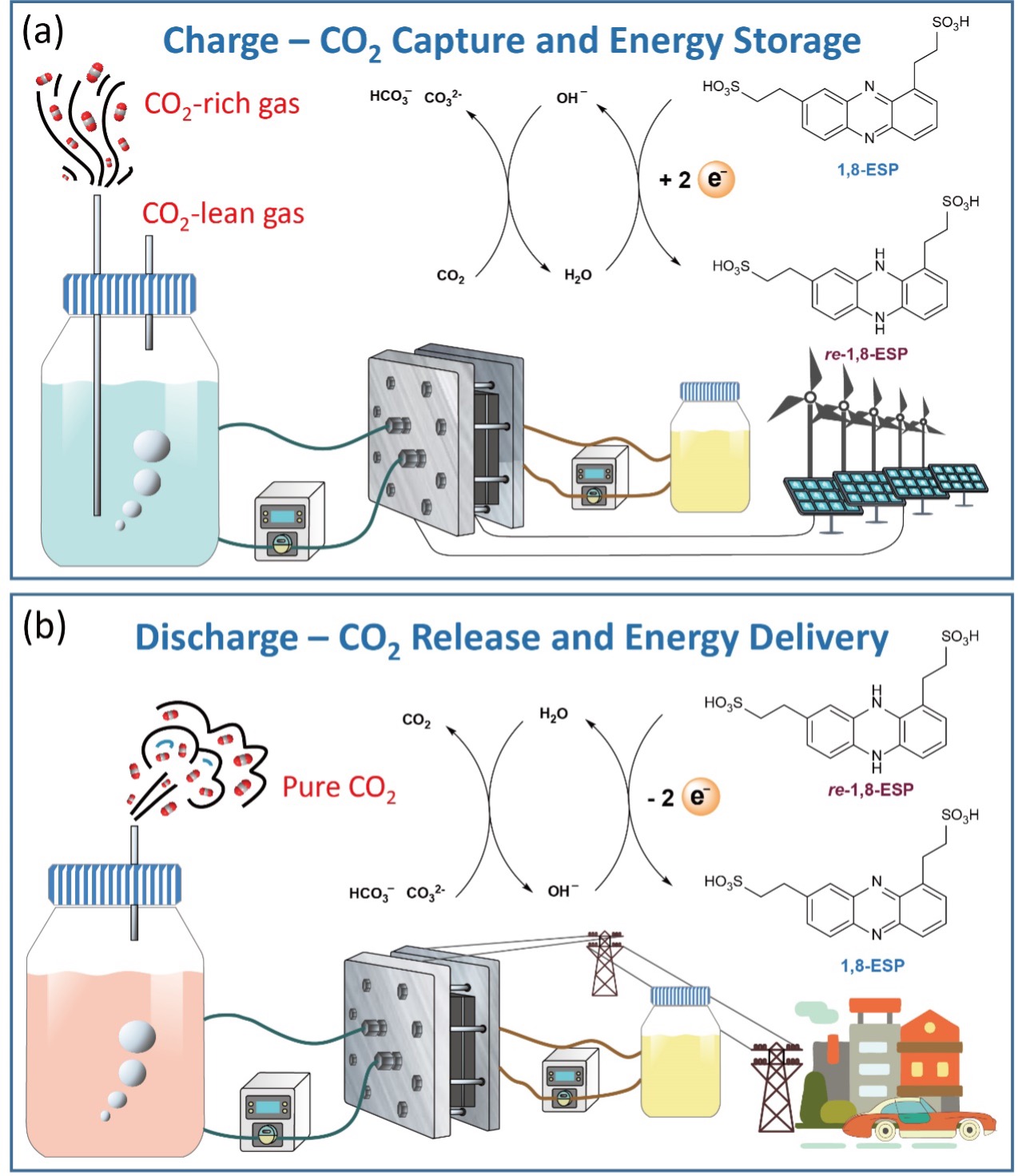
The newly developed molecule, 1,8-ESP, is easily soluble in water and has reversible electroactivity, fast kinetics, high chemical stability, and low permeation through membranes in cells. The 1,8-ESP cells offer high performance as pure energy storage devices when isolated from CO2, which enables operators to increase revenue by taking advantage of electricity price arbitrage when market conditions are suitable and implementing CO2 capture at other times. In addition, testing of the cells showed remarkably little degradation in performance over time, further confirming their commercial potential. These results show that 1,8-ESP can be the basis of high-performance systems for CO2 capture, energy storage, or both.
RELATED




















