




Search websites, locations, and people

CAP for Solar Fuels Researchers Propose New Strategy For Designing Advanced Methanation Catalysts under Mild Conditions
12, 2023
Email: sangeet@westlake.edu.cn
Phone: +86-(0) 571-88112035
Office of the Dean, School of Science
The Center of Artificial Photosynthesis (CAP) for Solar Fuels has made progress in methanation reaction, proposing a new mechanism for effective CO activation and hydrogenation based on density functional theory calculations and micro-kinetics modeling. Their study, titled “Toward Carbon Monoxide Methanation at Mild Conditions on Dual-Site Catalysts,” was published in the Journal of the American Chemical Society (J. Am. Chem. Soc. 2023, 145, DOI: 10.1021/jacs.3c02180).
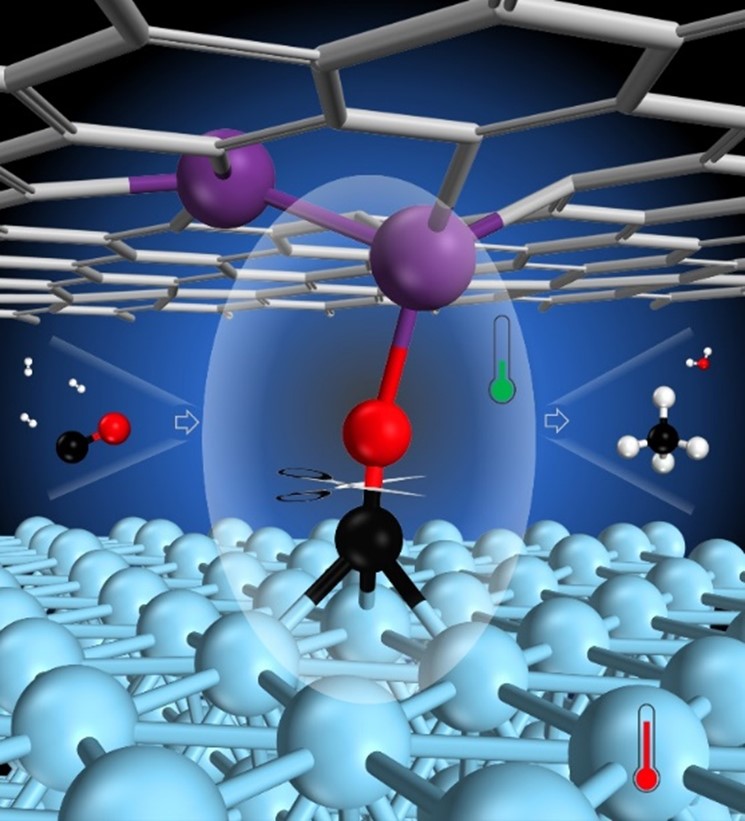
Since it was discovered by Sabatier and Senderens in 1902, the catalytic COx hydrogenation to methane (methanation) has served as an ideal model reaction for the fundamental understanding of catalysis on the gas-solid interface. This reaction plays an essential role in various industrial processes such as CH4 production, COx removal in hydrogen purification for fuel cells, and ammonia synthesis processes. The conversion of CO to methane is mainly limited by challenges associated with the breaking of the strong C-O bond and thus the reaction runs at high temperatures to overcome this barrier. Like the Haber-Bosch process, the methanation reaction is an exothermic reaction, and hence the increased total pressure is needed to shift to higher equilibrium conversion at elevated temperatures. Altogether, this will increase the energy consumption of the reaction, introduce higher demands on the pressure resistance of the reactor, and the temperature resistance of the catalyst, and also require efficient solutions for the heat transfer properties of the reactor. Therefore, developing a low-temperature solution for the methanation reaction is one of the key scientific challenges to be resolved and this clearly requires a more energy-efficient catalyst.
To address the aforementioned challenge, Dr. Tao Wang’s team proposed a dual-site strategy to achieve an effective CO activation. Based on their comprehensive DFT calculations for the CO hydrogenation mechanism to methane on stepped surfaces and confined dual-site for a group of transition metals, the confined dual-site generally shows more than 2 orders of magnitude higher methane TOF than undercoordinated step sites. The duality of the confined site enables the integration of two distinct active components to tune the activity even further – the proposed Co-Cr2/G catalyst is designed to achieve both facile CO dissociation as well as C/O hydrogenation, thus allowing the circumventing of the restrictions set by the linear scaling relations for CO methanation. The theoretically calculated TOFCH4 for the Co-Cr2/G dual-site catalyst is 4-6 orders of magnitude higher than what can be achieved for the Co(211) step sites. Clearly, such improvement in activity allows the operation of the methanation reaction under milder conditions and still achieves a similar TOF. The proposed confined dual-site strategy will help guide the design of new catalysts capable of activating inert chemical bonds at milder conditions, thus leading to more sustainable process solutions in heterogeneous catalysis.
Dr. Tao Wang, the PI of CAP for Solar Fuels and the Department of Chemistry at Westlake University, is the corresponding author, while Dr. Wanghui Zhao and Mr. Gaomou Xu are the first authors of this study. This work was financially supported by the National Natural Science Foundation of China, the National Key R&D Program of China, and the Research Center for Industries of the Future (RCIF) at Westlake University. The High-Performance Computing Center at Westlake University provided computational assistance.
RELATED




















