




Search websites, locations, and people

Causal AI Model Identifies New Combination Therapies for Cold Tumors
06, 2025
Email:
Phone:
Researchers from Westlake University, Caltech, and Cedars-Sinai Medical Center have developed a powerful machine-learning framework, Morpheus, that identifies molecular changes capable of transforming cold tumors-those lacking T cells-into hot tumors, which are heavily infiltrated by T cells and more responsive to immunotherapy. This work, published in Nature Biomedical Engineering, could help unlock new strategies for making immunotherapy more effective against tumors that have been difficult to treat, including colorectal cancer and breast cancer.
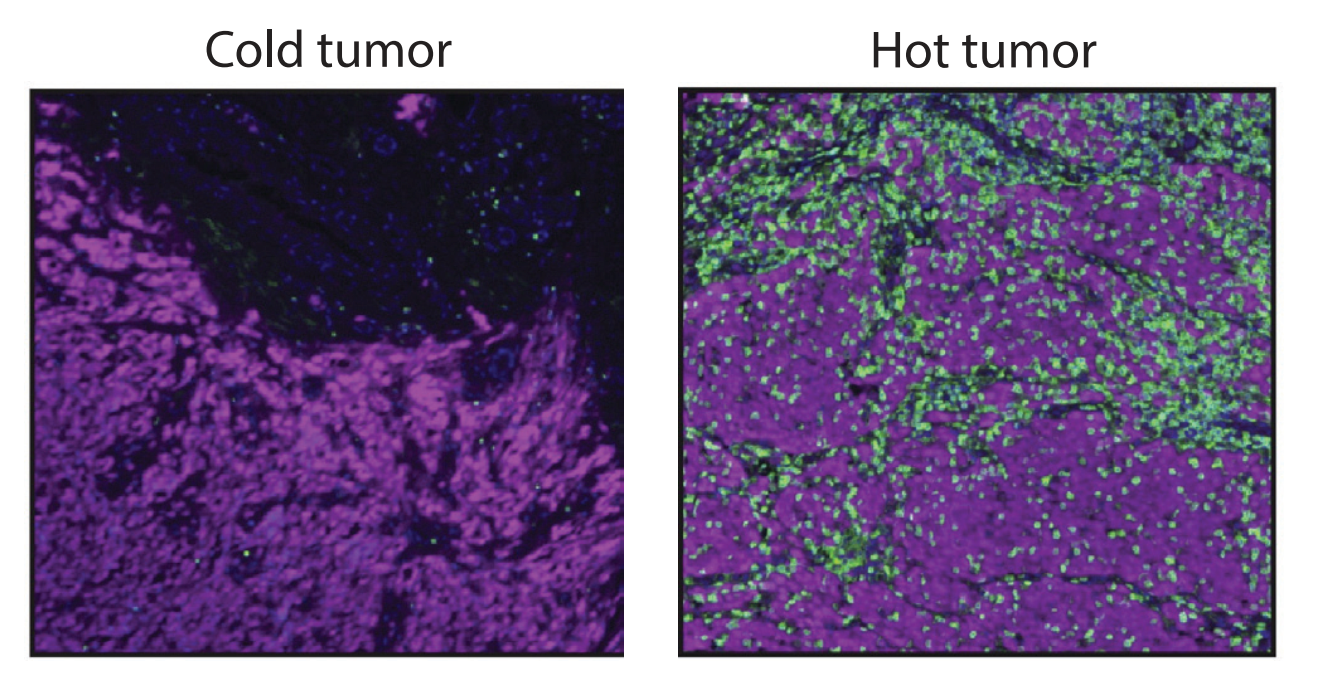
Figure 1: "Hot" versus "Cold" Tumors. Images of hot and cold tumors visualized by CD3+ T cells (green) and a tumor marker (magenta), images adopted from L. van der Woude et al. (2017).
While immunotherapy has transformed treatment for blood cancers, its effectiveness in solid tumors remains limited, with fewer than 20% of patients responding. A key challenge is that T cells, the immune system's key fighters, often fail to penetrate deep into tumor tissue due to immune-suppressing signals and physical barriers within the tumor microenvironment (TME). Scientists have long sought ways to reprogram these environments to support immune infiltration, but identifying the right combination of molecular targets has remained difficult.
A New Approach: Identifying Combinatorial Cancer Therapy with Causal AI
By Adara Koivula and Inna Strazhnik
Leveraging recent advances in spatial omics, high-resolution imaging techniques that map molecular signals within tumors, the researchers trained Morpheus to analyze large spatial proteomic datasets of tumor samples and predict minimal but effective molecular changes that could enhance T-cell infiltration. The key innovation in Morpheus is its use of \textbf{counterfactual reasoning}, a machine-learning technique often used to understand how AI models make decisions, such as why an AI algorithm rejected a loan application. Rather than just classifying tumors, Morpheus asks:
What minimal molecular changes would be needed to shift a tumor from cold to hot?
When Morpheus is fed a new cold tumor, it searches through its training data to find the most similar tumor microenvironment-except instead of being cold, this matched environment is already infiltrated by T cells. By comparing the two, Morpheus identifies a minimal set of molecular changes needed to transform the cold tumor into a hot one, making it more responsive to immunotherapy. In this way, Morpheus works like a skilled interior designer: given your home's layout and existing furniture, it suggests the most efficient adjustments-like repositioning a couch or changing the lighting-to maximize the aesthetic appeal of the space.
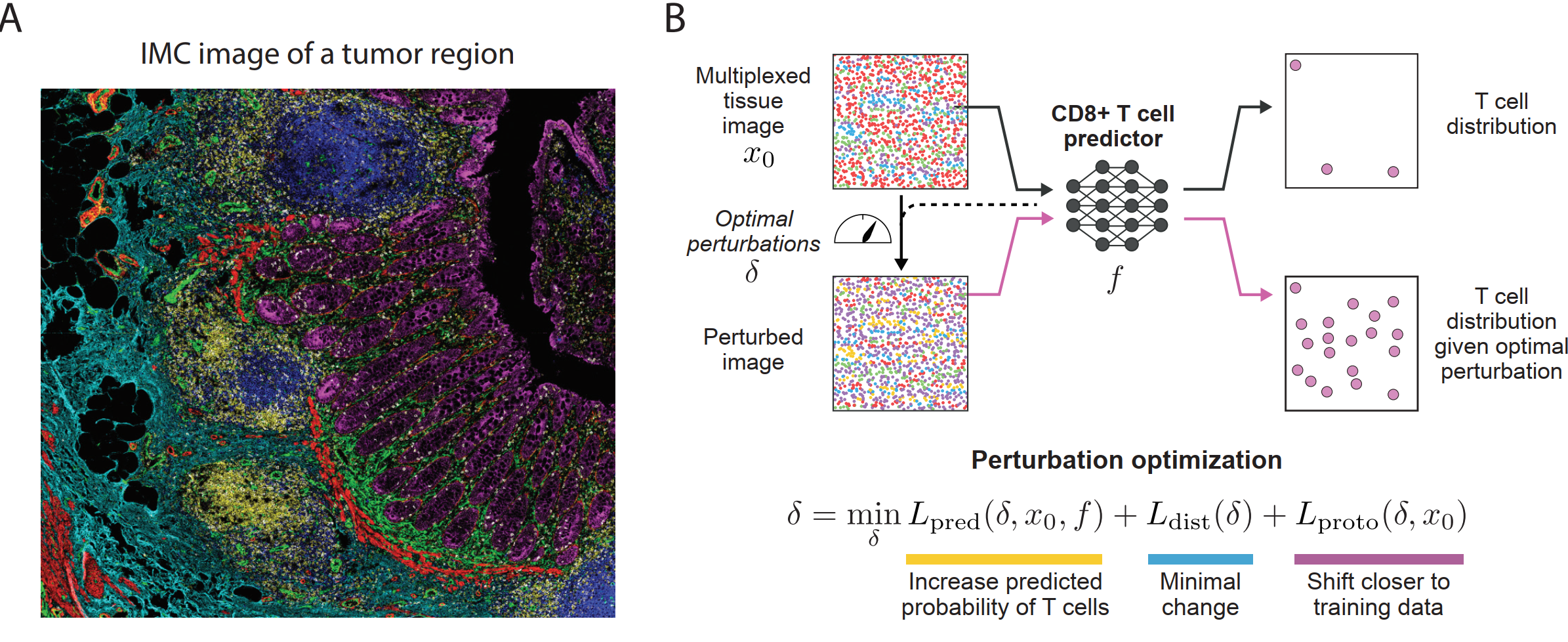
Figure 2: Overview of Morpheus framework. (A) Representative IMC image of a tumor slice used to train Morpheus, each color marks a specific protein. (B) Morpheus consists of first training a neural network classifier to predict the presence of CD8+ T cells from multiplexed tissue images where CD8+ T cells are masked, then we identify perturbation strategies that increase probability of T cells, require a small number of targets, and stay close to the data manifold.
By applying this approach to 368 imaging mass cytometry (IMC) images of tumor samples from patients with metastatic melanoma and colorectal cancer, Morpheus identified promising intervention strategies. For melanoma, it highlighted key molecular targets such as CXCL9, CXCL10, CCL18, and CCL22, while for colorectal cancer, it pinpointed PD-1, CXCR4, PD-L1, and CYR61. Crucially, Morpheus predicted that targeting combinations of these molecules--rather than single targets--would yield the most effective outcomes. To validate these predictions, researchers tested Morpheus' combinatorial therapies on human cancer and immune cells, demonstrating that these Morpheus-predicted combinations significantly outperformed standard monotherapies such as anti-PD-1 or anti-PD-L1.
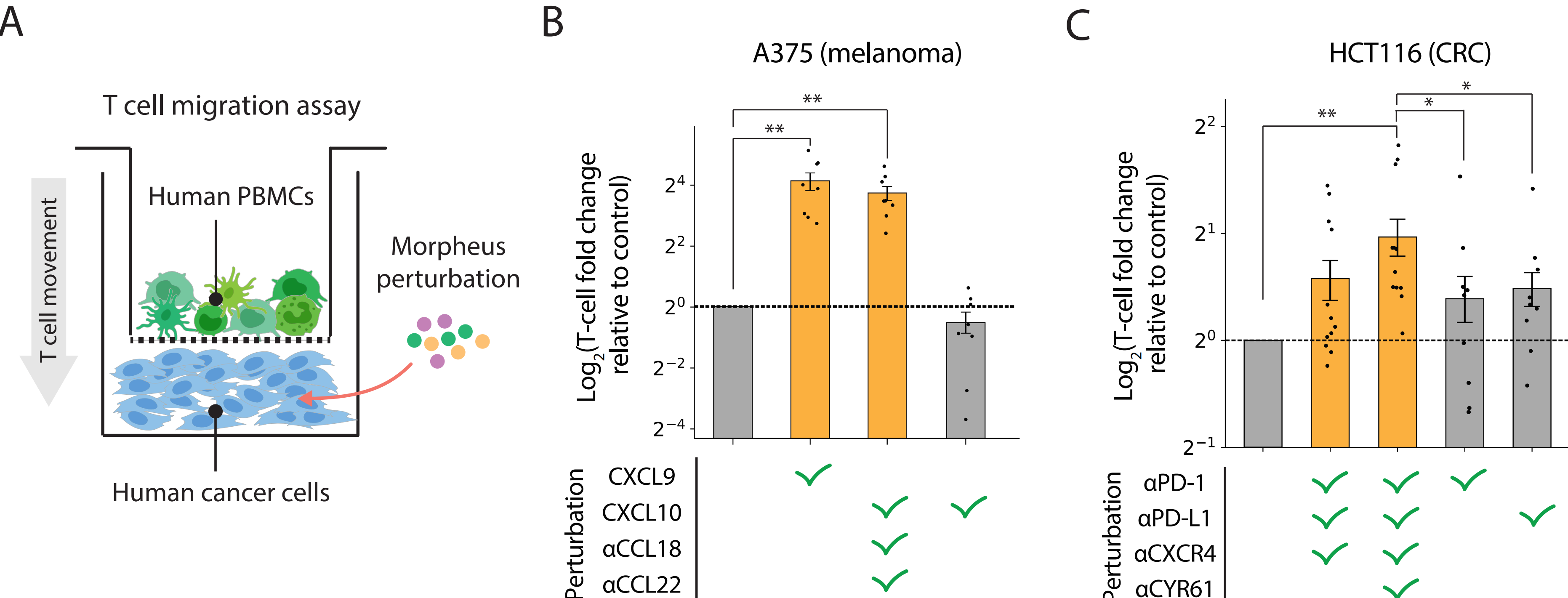
Figure 3: Experimental validation of Morpheus predictions. (A) experimental setup with results for (B) melanoma and (C) colorectal cancer, yellow bars indicate combination therapies identified by Morpheus.
A Step Toward Personalized Immunotherapy
Morpheus was also able to detect distinct molecular strategies for different patient groups based on factors like metabolic conditions. This suggests that precision immunotherapy-tailoring treatments to an individual's tumor profile-could become a reality with AI-powered predictions.
The power of Morpheus extends beyond cancer. Given the relevant diseased tissue, Morpheus could help design targeted therapies for autoimmune diseases, chronic infections, and fibrotic diseases, where immune cell infiltration and spatial organization play a crucial role in disease progression.
“By shifting from classification to intervention, we hope this work inspires the development of new models that not only classifies tumors but actively predict ways to reprogram them,'' said Dr. Zitong Jerry Wang, lead researcher on the study.
As spatial omics technology advances, Morpheus could serve as a blueprint for AI-driven strategies that transform large-scale tissue imaging data into actionable therapies.
What’s next?
While this study marks a significant step forward, several critical areas require further research. The researchers are working to obtain more tissue imaging data from a larger number of patients. In particular, integrating data from spatial transcriptomics platforms such as Xenium from 10x Genomics and Stereo-seq from BGI would enable Morpheus to analyze a broader set of genes, increasing the number of potential therapeutic targets. Additionally, the researchers are looking to test these therapies in experimental systems that more closely mimic human tumors, such as patient-derived xenografts in humanized mice. Since these insights are derived from human data, conventional animal models may not be informative.
Figure 4: Patient-derived xenografts in humanized mice more closely mimic human TME
This work was led by Dr. Zitong Jerry Wang, who leads the Cell Ethology Lab at Westlake University.
Co-senior authors include Matt Thomson, Professor of Computational Biology at Caltech, and Alex Xu, Assistant Professor at the University of Maryland (previously at Cedars-Sinai Medical Center). Other authors include Abdullah Farooq, Yu-Jen Chen, and Aman Bhargava.
For more details about ongoing research, visit https://cellethology.github.io/.
RELATED
Study at Westlake
University News




















