




Search websites, locations, and people

Researchers Develop Electrochemical Mitsunobu-Type Reaction with Broad Substrate Scope
26, 2024
Email:
Phone:
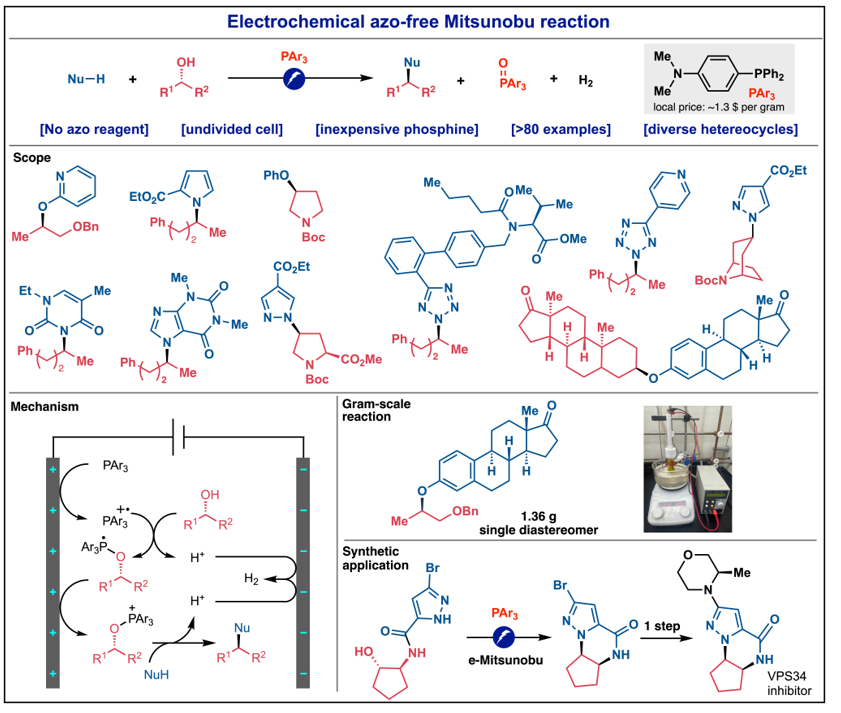
Prof. Pengfei Hu’s group has reported a sustainable electrochemical Mitsunobu-type reaction, featuring azo-free alcohol activation and broad substrate scope. This innovative method can be readily used in the modification of diverse bioactive natural products and multiple-step synthesis of a drug candidate.
The study, titled "Electrochemical Azo-free Mitsunobu-type Reaction," was recently published in Angew. Chem. Int. Ed. Professor Pengfei Hu from the Department of Chemistry at Westlake University is the corresponding author, and Dr. Quanping Guo, a postdoctoral fellow, is the first author of the study.

The classic chemical Mitsunobu reaction suffers from the need of excess alcohol activation reagents and the generation of significant by-products. Efforts to overcome these limitations have resulted in numerous creative solutions, but the substrate scope of these catalytic processes remains limited.
The user-friendly technology developed by Prof. Hu’s group tackles this issue as it features azo-free alcohol activation and broad substrate scope. Compared with other efforts towards sustainable Mitsunobu reaction, this reaction can be readily used to modify and synthesize diverse bioactive heterocyclic natural products and drug molecules derivatives.
Moreover, the simple undivided cell setup, the use of commercially available inexpensive electrodes, together with the scalability, highlight the practicability of this reaction.
(Scope of alcohol)
In the paper, the researchers highlight their novel reaction as an advancement towards achieving a fully catalytic Mitsunobu reaction, stating that their future work will focus on building upon this significant progress.
The study was funded by Westlake University and the National Natural Science Foundation of China (NSFC).
RELATED




















